Moderna Vaccine Wikipedia
The CVnCoV RNA vaccine from CureVac failed in. Based on evidence from clinical trials in people aged 18 years and older the Moderna vaccine was 941 effective at preventing laboratory-confirmed COVID-19 infection in people who received two doses and had no evidence of being previously infected.
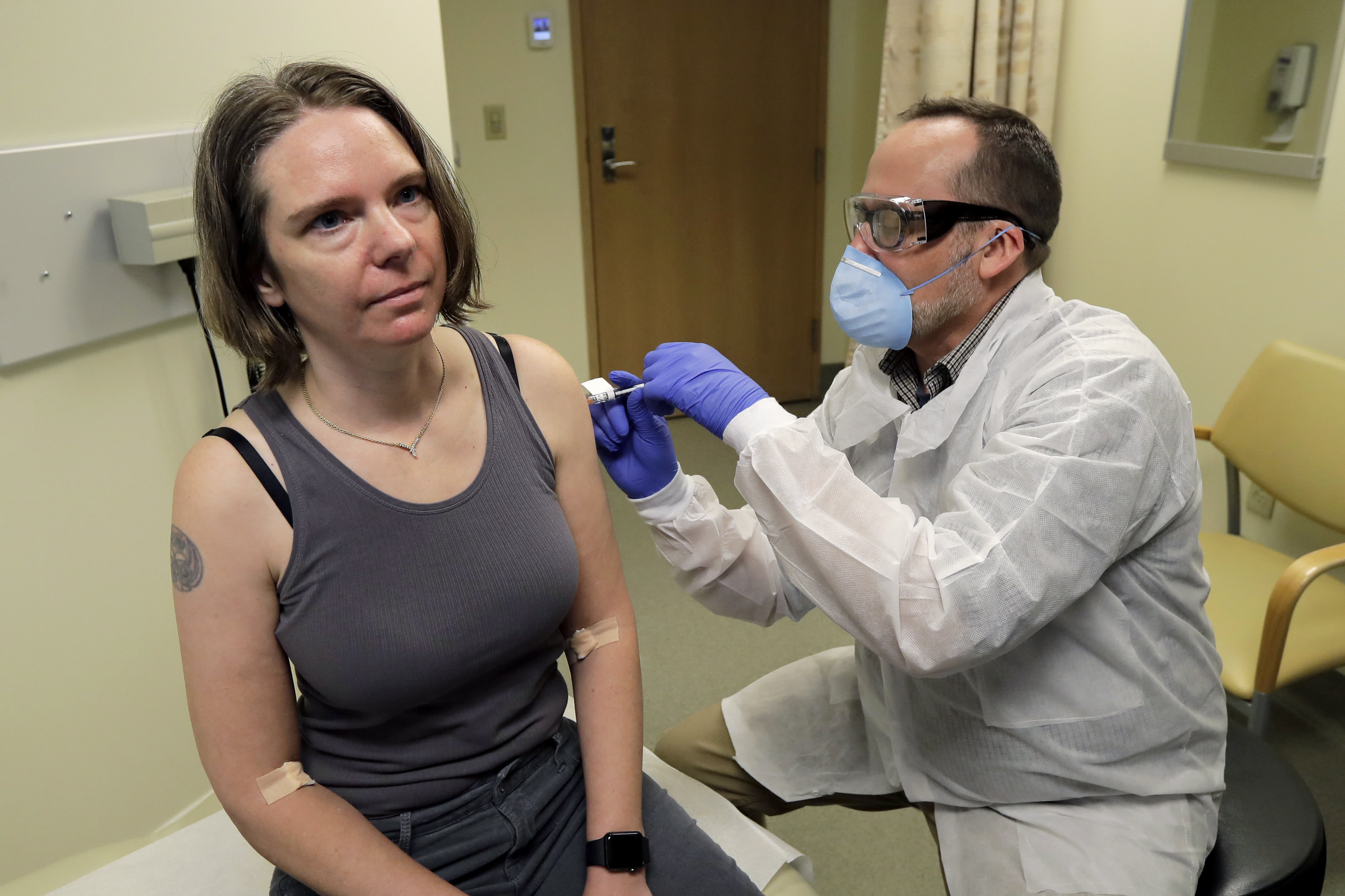
Moderna Shares Jump As Much As 16 After Company Says Its Coronavirus Vaccine Trial Produced Robust Immune Response
Moderna COVID-19 vaccine The Moderna COVID19 vaccine elasomeran codenamed mRNA-1273 and sold under the brand name Spikevax is a COVID-19 vaccine developed by Moderna the United States National Institute of Allergy and Infectious Diseases NIAID and the Biomedical Advanced Research and Development Authority BARDA.

Moderna vaccine wikipedia
. A 95 effective vaccine is better than a 62 to 90 effective vaccine but 200 million doses is better than 20 million to 50 million doses. Dr Robert Malone the creator of mRNA vaccine technology discussed the serious safety concerns associated with the Pfizer and Moderna vaccine for COVID-19 CHBV in a podcast for the Dark Horse YouTube Channel on 10 June with evolutionary biologist Bret Weinstein and tech entrepreneur Steve Kirsch which a Japanese. The Moderna fact sheet states. In short Pfizer and Moderna are producing fewer but more effective and pricier vaccines while AstraZeneca is making a greater number of less effective and cheaper vaccines.This Snapshot feature looks at the possible side effects and safety recommendations associated with. FDA Authorizes Up To 15-Doses Per Vial of its COVID-19 Vaccine. Two mRNA vaccines one by Pfizer and one by Moderna were approved late in 2020Both are over 90 effective. Moderna is a biotechnology company founded in 2010.
IG Farben is infamous for its mass production of. The Moderna COVID-19 vaccine is a two-dose vaccine to prevent COVID-19. Censored by Wikipedia over mRNA vaccine warnings. Fact Sheet for Healthcare Providers Administering Vaccine.
Severe allergic reactions Myocarditis inflammation of the heart muscle and Pericarditis inflammation of the lining outside the heart These may not be all the possible side effects of the Moderna COVID-19 Vaccine. Moderna was a pharmaceutical company that started in Germany under the name IG Farben. But Modernas first human trials arent so ambitious focusing instead on the crowded field of vaccines where the company has only been working since 2014. Every cell in your body uses mRNA which gives instructions to make the proteins your body needs.
RNA vaccines were the first COVID19 vaccines to be authorized in the United Kingdom the United States and the European Union. We refer to a modality as a family of potential mRNA medicines consistent both in design and how and where they are delivered in the body. The Moderna mRNA-1273 vaccine was authorized by the Food and Drug Administration for emergency use during the ongoing COVID-19 pandemic in December 20201 Out of 15185 participants in the phase III Coronavirus Efficacy COVE trials who received at least one dose of the Moderna vaccine 228 15 reported hypersensitivity adverse events including injection site rash and urticaria2. But there are a few key differences.
How Does It Work. A few vaccines also went into use in early 2021. A COVID-19 vaccine is any of the vaccines used against COVID-19 a disease caused by the SARS-CoV-2 virusIn July 2020 more than 150 vaccines were being developed in different laboratories. Instead they teach our cells how to make a proteinor even just a piece of a proteinthat triggers an immune response inside our bodies.
Moderna Provides Storage Update Announces the US. What is Moderna COVID-19 Vaccine EUA. Het Moderna-COVID-19-vaccin codenaam mRNA-1273 is een COVID-19-vaccin van het type mRNA-vaccin ontwikkeld door de Amerikaanse overheidsdiensten National Institute of Allergy and Infectious Diseases NIAID en Biomedical Advanced Research and Development Authority BARDA en het eveneens Amerikaanse farmaceutische bedrijf Moderna. Modalities may focus on different diseases within one therapeutic area - or across therapeutic areas - allowing us to potentially use one technological solution to create medicines for many diseases.
MRNA vaccines are a new type of vaccine to protect against infectious diseases. FDA Makes Two Revisions to Moderna COVID-19 Vaccine Emergency Use Authorization to Help Increase the Number of Vaccine Doses Available. To trigger an immune response many vaccines put a weakened or inactivated germ into our bodies. Modernas coronavirus vaccine is similar to the Pfizer-BioNTech vaccine that was authorized and shipped out to Americans earlier this week.
Authorized vaccines of this type are the PfizerBioNTech and Moderna vaccines. At Moderna we are pioneering a class of medicines based on messenger RNA mRNA.

File 201228 F Vs137 1002 Jpg Wikimedia Commons

Vaccino Anti Covid 19 Moderna Wikipedia

Pfizer Biontech Covid 19 Vaccine Wikipedia
Janssen Covid 19 Vaccine Wikipedia

Czech Republic Coronavirus Updates Jan 7 2021 Moderna Vaccine Approved And Enroute Prague Czech Republic
How The U S Government Bolstered Moderna S Covid 19 Vaccine Candidate Drug Discovery And Development

Komentar
Posting Komentar